How Many Protons Does Scandium Have
How many protons neutrons and electrons does gold have How many neutrons does scandium have?||number of neutrons in scandium Scandium summary
Selenium - Periodic Table and Atomic Properties
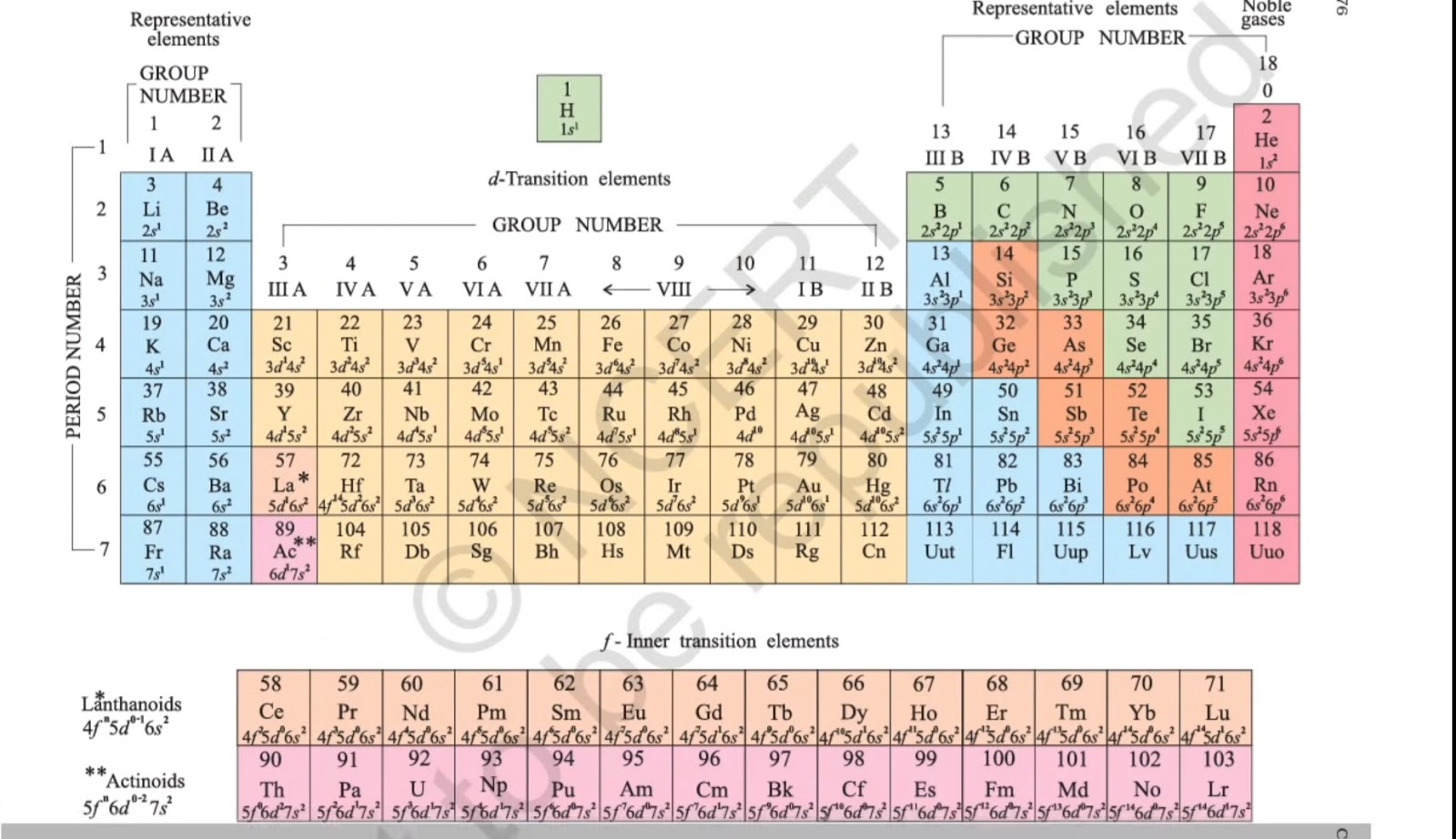
Scandium, atomic structure Calcium titanium vanadium scandium potassium atom manganese electron configuration element structure atomic shell number electronic facts ca symbol science heat How many neutrons does scandium have?||number of neutrons in scandium
Bohr scandium model atomic structure sc number diagram energy atom elements level first potassium
Neutrons protons electrons mass scandium atomic neon periodic chlorineNeutrons protons electrons neon chlorine potassium periodic calcium scandium numbers isotope Protons electrons neutrons elements nitrogen has argon neon list boron carbon2022: ☢️ valence electrons in scandium (sc) [& facts, color, discovery.
Electrons cobalt scandium atomic symbol number mass atom periodic table element shells protons electron square description chem4kids elements copper hasSymbol and electron diagram for scandium illustration stock vector Chem4kids.com: scandium: orbital and bonding infoScandium atom.

Scandium electron configuration libary 2s
Selenium titanium protons neutrons atomic scandium electrons number electron periodic table configuration properties mass radius elements electronegativity other density affinityCobalt structure atomic Periodic table of elements list with protons neutrons and electronsScandium periodic britannica.
How many neutrons does scandium have?||number of neutrons in scandiumChemical elements.com Scandium electron configuration photograph by animate4.com/scienceScandium neutrons does answer.

Protons neutrons & electrons of all elements (list + images)
Scandium electronScandium atomic structure sciencephoto Electrons protons neutrons fluorine atom employees csbsju atoms proprofs chemistry 10thCobalt, atomic structure.
Scandium valence electronsComplete an orbital diagram for scandium sc Protons neutrons electrons periodicScandium orbital bonding.